APOE*3-Leiden.CETP mouse
APOE*3-Leiden.CETP mouse
APOE*3-Leiden.CETP mouse
Translational model for Cardiovascular and Metabolic Diseases
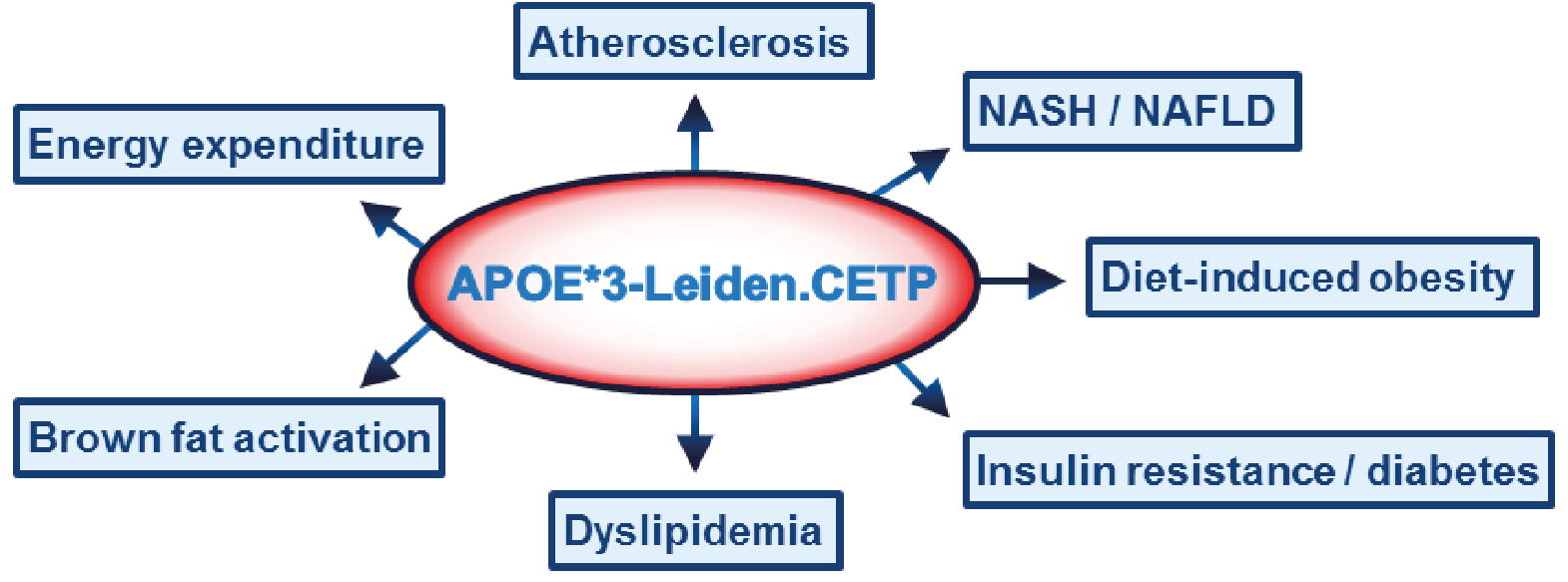
Leiden Metabolic Research Services (LMRS) provides a large contract research program on energy metabolism in relation to obesity, type 2 diabetes and atherosclerosis. Within this field of expertise we offer custom-made drug and dietary intervention studies in our unique APOE*3-Leiden.CETP mouse model, a well-established model for human-like lipid metabolism and atherosclerosis.
Main advantage over other mouse models
√ Responds to lipid-lowering and HDL-increasing interventions similarly as humans: accomplished by its intact expression of apoE and the LDL receptor, and transgenic expression of human CETP. Of note, apoE-deficient and LDL receptor-deficient mice do not respond to lipid-lowering interventions.
√ Responds to lipid-lowering and HDL-increasing interventions similarly as humans: accomplished by its intact expression of apoE and the LDL receptor, and transgenic expression of human CETP. Of note, apoE-deficient and LDL receptor-deficient mice do not respond to lipid-lowering interventions.
Human-like pathologies
√ Dyslipidemia, with plasma lipid levels easily titrated to desired levels.
√ Insulin resistance, which develops on high-fat diets.
√ Non-alcoholic steatohepatitis (NASH), with hepatic accumulation of lipids and inflammatory cells.
√ Atherosclerosis, driven by both cholesterol and inflammation.
√ Dyslipidemia, with plasma lipid levels easily titrated to desired levels.
√ Insulin resistance, which develops on high-fat diets.
√ Non-alcoholic steatohepatitis (NASH), with hepatic accumulation of lipids and inflammatory cells.
√ Atherosclerosis, driven by both cholesterol and inflammation.
Validated intervention strategies
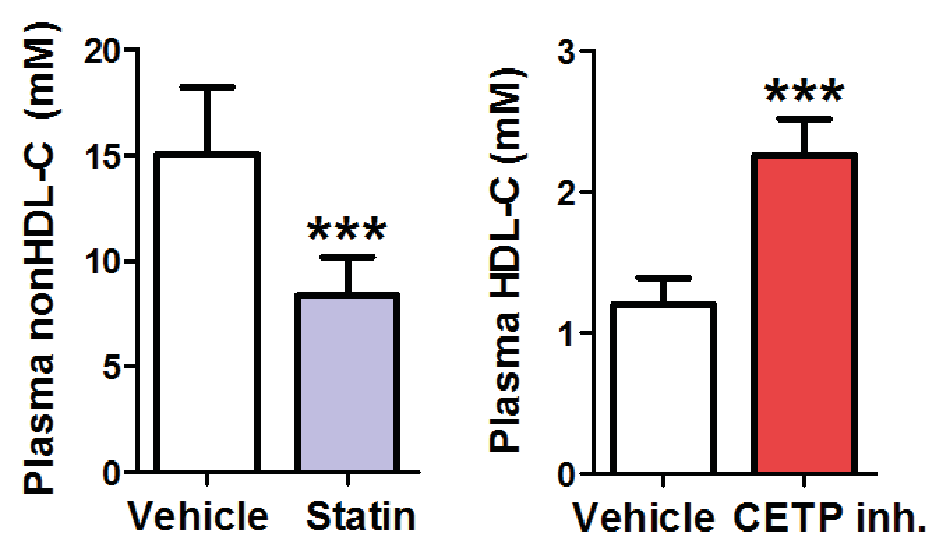
√ Lipid-lowering strategies, with reduction of atherosclerosis (e.g. statins, PCSK9 inhibitors, fibrates).
√ HDL-raising strategies, with reduction of atherosclerosis (e.g. CETP inhibitors, niacin).
√ Anti-diabetic strategies, with improvement of insulin sensitivity (e.g. GLP1 analogs, rosiglitazone).
√ Lipid-lowering strategies, with reduction of atherosclerosis (e.g. statins, PCSK9 inhibitors, fibrates).
√ HDL-raising strategies, with reduction of atherosclerosis (e.g. CETP inhibitors, niacin).
√ Anti-diabetic strategies, with improvement of insulin sensitivity (e.g. GLP1 analogs, rosiglitazone).
Dyslipidemia
APOE*3-Leiden.CETP mice have a human-like plasma lipoprotein profile and plasma lipid levels. These mice respond to lipid-lowering strategies by virtue of a functional hepatic lipoprotein remnant clearance pathway involving apoE and LDLr. Moreover, as they express human CETP they also respond to HDL-increasing strategies. This is thus an ideal model for pharmacological and dietary interventions for treatment of dyslipidemia.
Atherosclerosis
APOE*3-Leiden.CETP mice develop diet-induced atherosclerotic lesions with all characteristics of human lesions. The various stages of atherosclerotic lesions (type I to V) can be distinguished and develop within 3-6 months, depending on the dietary cholesterol content. Numerous pharmacological and dietary intervention studies have been
performed in this model investigating the effect on atherosclerosis progression and regression.
performed in this model investigating the effect on atherosclerosis progression and regression.
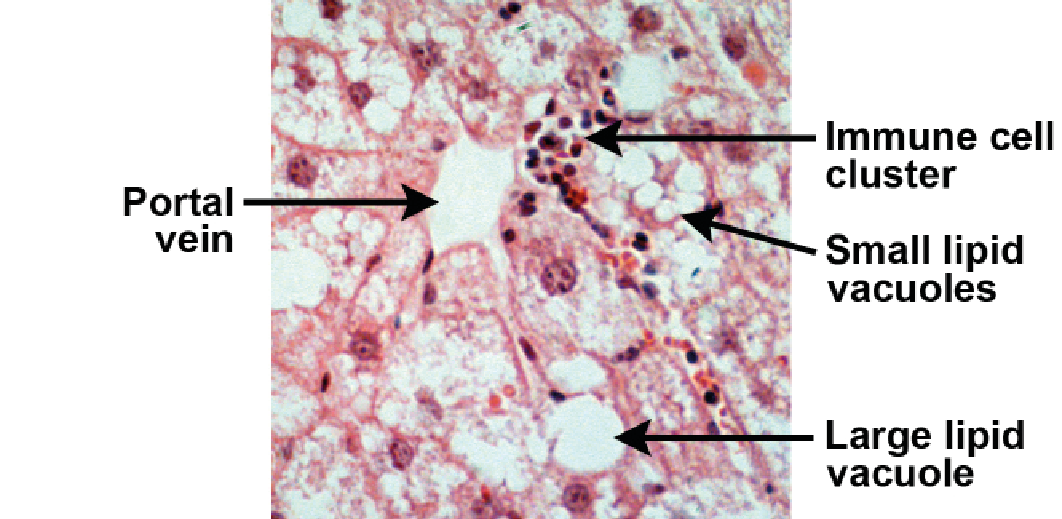
Nonalcoholic steatohepatitis
APOE*3-Leiden.CETP mice develop nonalcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) when fed a high-fat or Western-type diet. Typical characteristics resembling human NASH are observed, including macrophage
and neutrophil infiltration and hypertrophy. This allows efficacy studies of compounds and dietary interventions aimed at reducing NASH in a representative, human-like setting.
and neutrophil infiltration and hypertrophy. This allows efficacy studies of compounds and dietary interventions aimed at reducing NASH in a representative, human-like setting.
Obesity and insulin resistance
APOE*3-Leiden.CETP mice develop high-fat diet-induced obesity and insulin resistance. We offer custom-made drug and dietary intervention studies on all aspects of obesity and insulin resistance. The hyperinsulinemic euglycemic clamp analysis, the golden standard for insulin sensitivity, allows studying insulin sensitivity as well as glucose and fatty acid metabolism in various organs and tissues. Moreover, surrogate measures of insulin sensitivity such as glucose tolerance test are routinely performed. We additionally use genetic models of obesity, such as ob/ob and db/db mice.
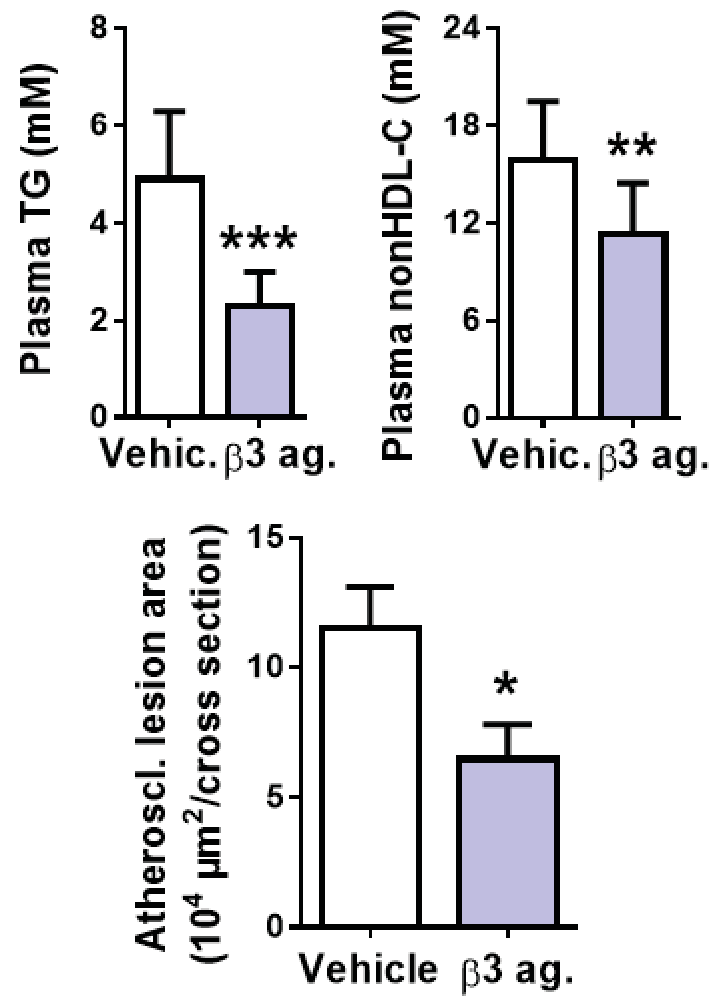
Energy expenditure and brown fat activation
Novel strategies to reduce hyperlipidemia, obesity, insulin resistance and atherosclerosis include increasing brown
fat activity. We offer a full set of in vivo and ex vivo techniques for investigation of energy expenditure, brown fat activity as well as mechanisms of action, including fully automated metabolic cages for determining glucose and fatty acid oxidation, and lipoprotein kinetics using tracers. In view of its intact apoE-LDLr-mediated remnant clearance pathway, the APOE*3-Leiden.CETP model is the preferred model as compared to other mouse models for studying the effect of brown fat modulation on hyperlipidemia and atherosclerosis.
fat activity. We offer a full set of in vivo and ex vivo techniques for investigation of energy expenditure, brown fat activity as well as mechanisms of action, including fully automated metabolic cages for determining glucose and fatty acid oxidation, and lipoprotein kinetics using tracers. In view of its intact apoE-LDLr-mediated remnant clearance pathway, the APOE*3-Leiden.CETP model is the preferred model as compared to other mouse models for studying the effect of brown fat modulation on hyperlipidemia and atherosclerosis.
Selected references
- Schilperoort M et al. Alternating light-dark cycles aggravate atherosclerosis in APOE*3-Leiden.CETP mice. J Pineal Res 2020; 68: e12614. 18.
- Van den Berg R et al. A diurnal rhythm in brown adipose tissue causes rapid clearance and combustion of plasma lipids at wakening. Cell Rep 2018; 22: 3521-3533.
- Van der Tuin SJ et al. Lipopolysaccharide lowers cholesteryl ester transfer protein by activating F4/80+Clec4f+Vsig4+Ly6C- Kupffer cell subsets. J Am Heart Assoc 2018; 7: e008105.
- Li Z et al. Butyrate reduces appetite and activates brown adipose tissue in mice via the gut-brain neural circuit. Gut 2018; 67: 1269-1279.
- Hoeke G et al. Atorvastatin accelerates clearance of lipoprotein remnants generated by activated brown fat to further reduce hypercholesterolemia and atherosclerosis. Atherosclerosis 2017; 267: 116-126.
- Berbée JFP et al. Deuterium-reinforced polyunsaturated fatty acids protect against atherosclerosis by lowering lipid peroxidation and hypercholesterolemia. Atherosclerosis 2017; 264: 100-107.
- Bartelt A et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat Commun 2017; 8: 15010.
- Van der Tuin SJL et al. Anacetrapib reduces (V)LDL-cholesterol by inhibition of CETP activity and reduction of plasma PCSK9. J Lipid Res 2015; 56: 2085-2093.
- Wang Y et al. Plasma cholesteryl ester transfer protein is predominantly derived from Kupffer cells. Hepatology 2015; 62: 1710-1722.
- Berbée JFP et al. Brown fat activation reduces hypercholesterolemia and protects from atherosclerosis development. Nat Commun 2015; 6:6356, doi 10.1038/ncomms7356.
- Kühnast S et al. Anacetrapib reduces progression of atherosclerosis, mainly by reducing non-HDL-cholesterol, improves lesion stability and adds to the beneficial effects of atorvastatin. Eur Heart J 2014; 36(1): 39-50.
- Kühnast S et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res 2014; 55(10): 2103-12.
- Wang Y et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br J Pharmacol 2014; 171(3): 723-34.
- Liang W et al. Metabolically induced liver inflammation leads to NASH and differs from LPS- or IL-1-induced chronic inflammation. Lab Invest 2014; 94(5): 491-502.
- Kooijman S et al. Inhibition of the central melanocortin system decreases brown adipose tissue activity. J Lipid Res 2014; 55(10): 2022-32.
- Geerling JJ et al. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes 2014; 63(3): 880-9.
- Van den Hoek AM et al. APOE*3-Leiden.CETP transgenic mice as model for pharmaceutical treatment of the metabolic syndrome. Diab Obes Metab 2014; 16(6): 537-44.
- Boon MR et al. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One 2013; 8(9): e74083.
- Kühnast S et al. Niacin reduces atherosclerosis development in APOE*3-Leiden.CETP mice mainly by reducing NonHDL-cholesterol. PLoS One 2013; 8(6): e66467.
- Bijland S et al. Fenofibrate increases very low density lipoprotein triglyceride production despite reducing plasma triglyceride levels in APOE*3-Leiden.CETP mice. J Biol Chem 2010; 285(33): 25168-75.
- Parlevliet ET et al. CNTO736, a novel glucagon-like peptide-1 receptor agonist, ameliorates insulin resistance and inhibits very low-density lipoprotein production in high-fat-fed mice. J Pharm Exp Ther 2009; 328(1): 240-8.
- De Haan W et al. Torcetrapib does not reduce atherosclerosis beyond atorvastatin and induces more proinflammatory lesions than atorvastatin. Circulation 2008; 117(19): 2515-22.
- Zadelaar S et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol 2007; 27(8): 1706-21. (Review)
- Zadelaar AS et al. Dual PPARalpha/gamma agonist tesaglitazar reduces atherosclerosis in insulin-resistant and hypercholesterolemic APOE*3-Leiden mice. Arterioscler Thromb Vasc Biol 2006; 26(11): 2560-6.
- Van Vlijmen BJ et al. Diet-induced hyperlipoproteinemia and atherosclerosis in APOE*3-Leiden transgenic mice. J Clin Invest 1994; 93(4): 1403-10.